Lithium-Ion Batteries and Gas Detection

Table of Contents
What Are Lithium-Ion Batteries?
Development and Applications
Used in everything from smart phones to tablets, television remotes, cars and more, batteries are an essential part of our daily modern life.
While there are many kinds of batteries, one type of battery which has been used in an increasing number of applications and whose production has increased significantly in recent years is the lithium-ion battery.
Lithium-ion batteries are secondary cell batteries which consist of an anode and a cathode and are charged and discharged as lithium ions move between them.
Easily confused with the similarly named lithium batteries, they are in fact two completely different types of batteries. Lithium batteries are simply disposable primary cell batteries made from the metal lithium.
The development of lithium-ion batteries was nothing short of revolutionary. During the 1980s, the development of portable devices such as mobile phones and laptop computers led to increased demand for high capacity yet compact lightweight secondary cell batteries.
This resulted in the widespread application of lithium-ion batteries, which had a higher energy density and were more compact and lightweight compared to conventional lead-acid and nickel metal hydride batteries used at the time.
Lithium-ion batteries are a technological innovation which allowed portable devices to be used for extended periods of time, contributing to the widespread popularity of mobile electronic devices.
This has allowed communications technology to become increasingly convenient, transforming the way we live and do business.
Lithium-ion batteries are also used to power so-called eco-friendly vehicles (EVs, HEVs, and PHEVs) and to power electrical systems in the aviation sector, making a significant contribution to the transportation industry as well.
Advantages
Some of the main advantages of lithium-ion batteries include their compact size and large energy storage capacity.
Their high internal voltage gives them high energy density, allowing for faster charging and discharging times.
For comparison, the energy density of lead-acid batteries ranges from 25 to 50 Wh/kg, whereas the energy density of lithium-ion batteries ranges from 50 to 260 Wh/kg. Lithium-ion batteries are also exceptionally durable for a longer useful life.
Widespread use of lithium-ion batteries is likewise leading to the “electrification” of society; that is, replacing the use of fossil fuels such as oil and gas with electric alternatives, an important part of the fight against climate change.
Moreover, lithium-ion batteries do not contain substances which place a significant burden on the environment such as cadmium, lead or mercury, making them an environmentally friendly alternative.
Disadvantages
The main disadvantage of lithium-ion batteries is that they can pose a fire risk.
If a lithium-ion battery’s two electrodes come into direct contact due to external pressure or for any other reason, this could result in a chemical reaction which gives off oxygen and heat, causing the flammable electrolyte to catch fire.
Because lithium-ion batteries have a higher energy density compared to other kinds of batteries, they run a higher risk of overheating, explosion or fire when overcharged, over-discharged or otherwise not used properly.
Gas Detection in Lithium-Ion Battery Manufacturing
Lithium-ion batteries are composed of an anode and a cathode divided by a separator filled with electrolyte.
During electrode manufacturing, a slurry consisting of active materials is applied to both sides of the aluminum foil used in the cathode and the copper foil used in the anode, which is then dried and pressed to achieve the desired density, resulting in electrode sheets.
Next, during cell assembly, a porous insulating film that allows ions to move is placed between the cathode and anode. The cathode, anode and insulating film are then layered and wound into a jelly roll.
After cell assembly is complete, the cell is filled with an electrolyte solution and then charged and discharged to stabilize the electrode materials.
Cells then undergo the aging, quality control and packaging processes, after which they are ready for shipment. In lithium-ion battery manufacturing, gas detection is necessary throughout the following processes and for the following target gases:
- Electrode Manufacturing: NMP, which is used as a solvent, is vaporized during the process of coating metal foils with slurry and during the drying process. Since nitrogen purge is partially used in this process, it is also necessary to detect nitrogen.
- Electrolyte Filling: If the electrolyte comes into contact with water during the filling process, this can result in hydrolysis and the release of harmful gases.
- Charging and Discharging: The chemical reactions which take place during repeated charging and discharging can release hydrogen and carbon monoxide.
- Storage: Changing temperatures throughout the year may result in changes to the environment in which batteries are stored. These changes can pose a risk of ignition. Because hydrogen is released prior to ignition, early detection is essential.
- Quality Control and Performance Testing: When batteries undergo severe and destructive testing processes, they may break or become damaged, causing electrolyte to leak from the battery and resulting in thermal decomposition or combustion of its components. This can potentially allow carbon monoxide or hydrogen to escape from within the battery.
Gas-Related Hazards
Gas detection plays a crucial role in ensuring worker safety during the lithium-ion battery manufacturing process.
Both the hazards present as well as the concentration levels and detection methods used vary significantly depending on the type of gas at hand.
We would therefore like to take this opportunity to provide a quick overview of the properties of some of the gases most frequently released during the process of manufacturing lithium-ion batteries.
- Hydrogen: Molecules of hydrogen gas are smaller than all other gases, making hydrogen particularly prone to gas leaks. A gaseous mixture consisting of 5% oxygen or more and 4% hydrogen or more can result in a hydrogen explosion when ignited.
- VOCs: Both electrolyte and the NMP used in electrode manufacturing are flammable organic solvents which can result in explosion when vaporized and found in high concentrations in their gaseous state.
- Nitrogen: When nitrogen gas is inhaled in high concentrations, it can result in oxygen deficiency and a loss of consciousness.
- Carbon monoxide: Inhaling even minuscule amounts can result in carbon monoxide poisoning, sometimes even resulting in death. Detecting carbon monoxide as early as possible is a fundamental part of ensuring safety in the work environment.
Challenges in Lithium-Ion Battery Manufacturing
Electrode Manufacturing and NMP
NMP is used as a solvent during the process of manufacturing lithium-ion battery electrodes.
Its high flash point of approximately 99℃ and boiling point of approximately 202℃ make it a relatively stable substance when used at room temperature.
When used during electrode manufacturing, however, the high temperature (120-130℃) drying process can cause NMP to vaporize and pose an explosion risk.
It is therefore necessary to regulate NMP levels to ensure that its concentration within the drying oven exhaust duct remains below its lower explosive limit of 1.3 vol% at all times.
NMP is flammable, necessitating the use of explosion-proof equipment that can withstand high temperature environments and ensure that it does not come into contact with sparks or any other source of flame.
Our Solution: SD-2500
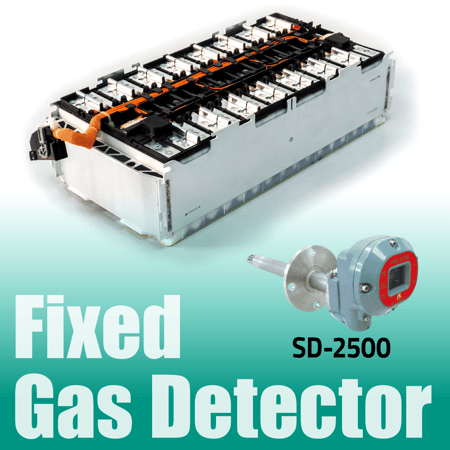
Gas monitoring plays an essential role in the process of manufacturing the electrodes used in lithium-ion batteries by keeping NMP concentrations under control and preventing explosions.
Our Riken Keiki SD-2500 fixed multi-gas detector has an ATEX/IECEx/UL-approved explosion-proof construction designed to withstand elevated temperatures and high gas concentrations and can be inserted directly into the drying oven duct.
In addition, the SD-2500 is designed to minimize sensor poisoning from silicon dioxide (SiO2). This is important as silicon dioxide emerges as a promising new anode material for lithium-ion batteries.
In addition to its outstanding durability, the SD-2500 is a diffusion-type detector which does not require the use of pumps or sampling tubes, translating into lower running costs when compared to suction-type gas detection equipment.

Want to hear from an expert?


You may also be
interested in

The Importance and Key Considerations of IP Codes When Choosing Gas Detectors
Learn why IP codes matter when choosing gas detectors. Discover how dust and water resistance ensure reliable performance and worker safety in harsh environments.

Heavy Fuel Oil and LNG
Heavy fuel oil powers global shipping with efficiency, affordability, and safety—maximizing cargo space and enabling long-distance voyages.

Gas Monitoring for a More Sustainable Society
Riken Keiki aids sustainability with gas monitors, supporting circular economy efforts and eco-friendly manufacturing practices.
Publications





